At a Glance
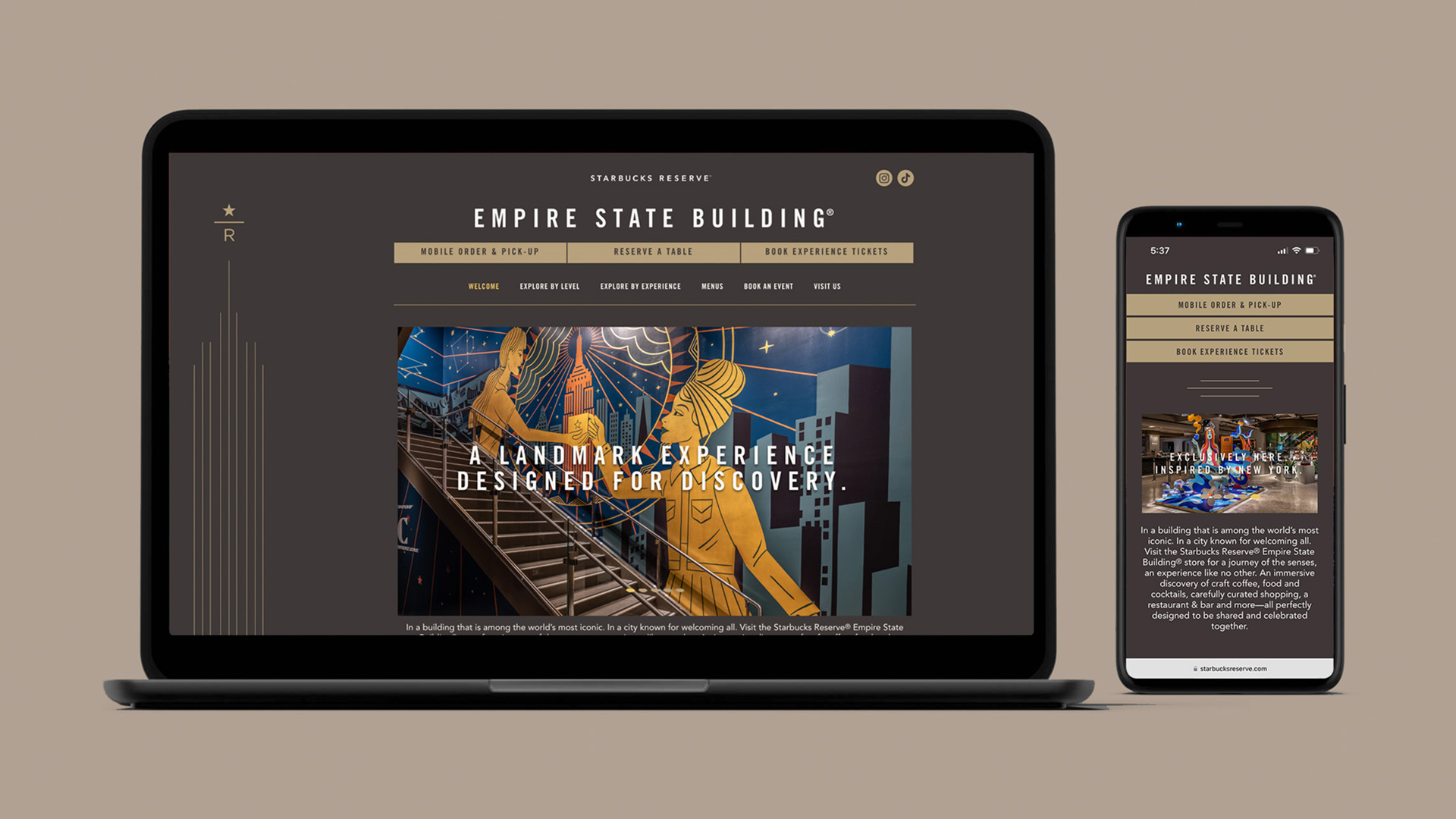
For 14 years, Aquent Studios has played an instrumental role in the marketing evolution at Merck. It's a powerful synergy that's transformed the content creation process, helping Merck achieve new heights of efficiency and innovation.
Capabilities Engaged
- Content Operations
- Content Stewardship
- Graphic Design
- Program Management
- Project Management

Project Overview
Challenge
Marketing and content operations at a pharma giant like Merck is a science in and of itself. The intricate medical, legal, and regulatory review process (MLR review) and complexities involved with content management for 30+ brands and multiple agency vendors demand precise referencing, annotation consistency, and adherence to strict regulatory requirements. Inadequate documentation leads to costly delays in the creation and approval of marketing content. The challenge was to identify gaps in the existing processes and create workflows that aligned with new requirements, especially in the context of agile teams, MLR review times, and changing regulatory landscapes.
Approach
Leveraging its deep pharma industry knowledge, Aquent Studios' strategic approach has involved a combination of specialized functions, meticulous oversight of the standardization process, data-driven analysis, and operational expertise to significantly improve the efficiency of the MLR review and content management processes at Merck.

What We Did
Marketing & Creative Operations Consulting
Identified the impact of inadequate referencing on efficiency and costs
Conducted a thorough analysis showcasing the impact of reference-related challenges on efficiency and costs. This data-driven approach clearly illustrated the need for standardized practices and the benefits of improved content management.
Process & Workflow Optimization
Developed improved content workflows and trained Merck teams and agencies on all new processes
Ensured compliance and managed the overall content creation process by unifying and documenting workflows. This involved recognizing gaps, streamlining processes, and providing ongoing training to Merck and its agencies of record.
Introduced new functions with specialized expertise in referencing and annotations
Created specialized roles to help combat inefficiencies, including Reference Stewards who oversee and manage references to ensure standardized practices in naming and annotations. Additionally, Design Stewards, Program Managers, and Content Series Program Managers facilitate the efficient movement through each phase of content production, ensuring compliance with regulatory requirements.
Content Operations & Delivery
Enabled faster content delivery and more projects to be completed by realizing new efficiencies
Completed over 4,000 projects annually, spanning every brand within Merck and touching virtually every aspect of content creation. We also partnered on the implementation and management of an Accelerated Approval Program for faster time to market.